I have been taught this Pregnenolone Steal/Cortisol Shunt thing-a-ma-bobber easily 100 times in continuing education material. I have taught it myself. It makes sense. We can wrap a nice little bow around it and pound it home. I’m not mad about it. The simplicity of the message helps people.
Yet, as a researcher I can’t just swallow everything whole that comes out of the Functional Medicine realm, I have to gnaw on it and spit out the parts that don’t taste right.
At times, I’m at conferences and the speaker will state something as fact that I absolutely know cannot be said in this manner and they lose me and everything they say is now shrouded in doubt.
Like this one, I hear in about every talk on male health “Ejaculations deplete zinc – zinc deficiency lowers testosterone (inference – masturbation lowers testosterone).” Show me an RCT on pubmed where the researchers had men ejaculate at different frequencies, controlled their dietary intake of zinc, and then measured zinc (can’t do it – no good measure) and hormone status at different follow-up intervals. Mechanistically, it could be possible as ejaculate does contain large amounts of zinc, but this zinc is derived from prostate stores, not whole body stores. NIH isn’t going to fund this trial, but if you want to fund me, I am sure I can find the dudes to make this study happen. I don’t even know if we would need incentives.
You see the problem. In Functional Medicine, it is easy to propagate myths or pseudoscience, especially if it sounds warm and fuzzy and feasible. Many of the lecturers and professionals have not done primary research or been taught how to pick apart stats or research design. This is not their fault and there are exceptions to every rule and no judgement. We are all learning, myself included. Yet, some seem to flat out not care about PubMed and even dismiss the scientific method. They run everything on anecdotal evidence and present their data as fact. I looked back at many of the handouts and material I was given on this “Pregnenolone Steal” and most do not have a single scientific reference and this cortisol shunt was presented as foundational material. Not good, and if I am wrong – please prove it. I want you to be right. This article is more of a discussion than a declarative informational post.
This mindset is so dangerous because although anecdotal evidence is a great way to drive research questions, it is not the end of the line. If we as a Functional Medicine community do this, it allows cracks of quackery into a field I care for dearly. We are under more and more scrutiny from the general public and researchers, thus we must strike a balance and I believe do research ourselves to drive the field forward. Furthermore, the rigidity of being completely cemented in PubMed is perhaps just as dangerous, but we have to do everything in our power to live on both sides of the fence.
It’s a dance and you can’t be inventing your own music.
Back to this idea of the “Pregnenolone Steal”, which is the ideology that when the body is stressed it diverts pregnenolone away from anabolic hormones and into cortisol which is catabolic in excess. This intuitively makes sense, if you are running from a tiger you are not going to take a little break to ovulate or put some extra inches on your biceps.
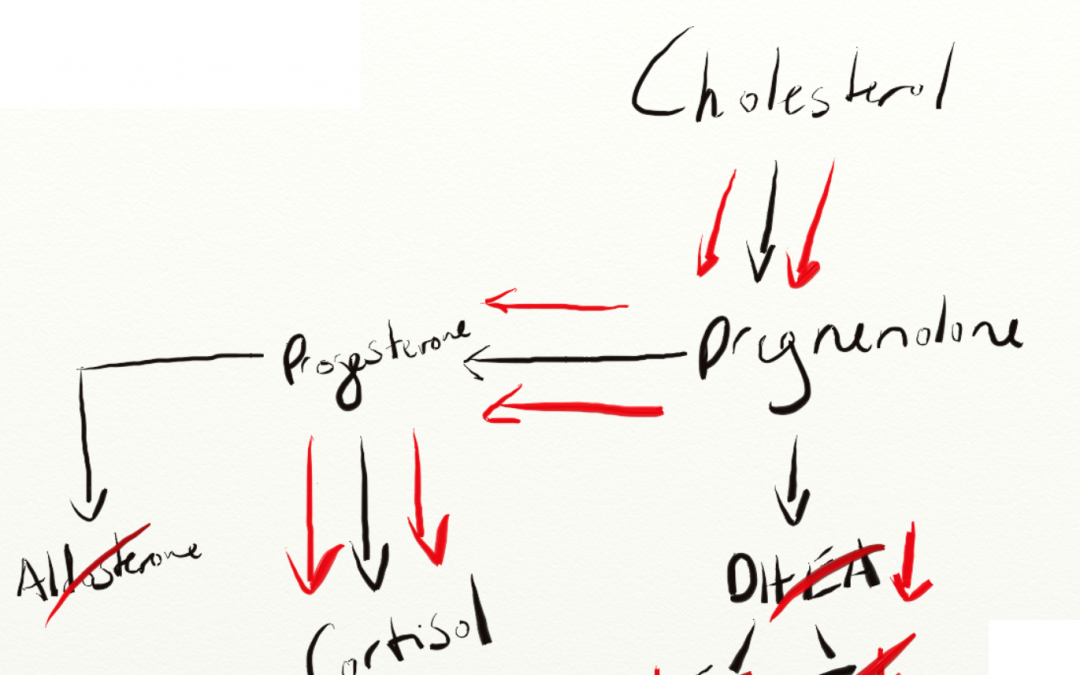
Here’s an image from a lab company that makes money off running salivary panels measuring these types of hormones.
The theory stands on a bit of solid ground as DHEA, Aldosterone, and Cortisol are all primarily synthesized in the adrenal cortex (however in different layers) and will compete for the same precursor – Pregnenolone.
But, let’s get into that a little bit.
The cytochrome P450 side-chain cleavage (P450scc) enzyme converts cholesterol to pregnenolone.
The above reaction takes place in the mitochondria and StAR (steroidogenic acute regulatory protein) is the substance that moves cholesterol into the mitochondria to start this whole party (LH and ACTH both upregulate StAR). (Interestingly, in rats, StAR production is inhibited by ethanol consumption.)
“The StAR/P450scc system is rate-limiting and hormonally regulated; hence, this step is the quantitative regulator of steroidogenesis. By contrast, P450c17 is the qualitative regulator, determining which class of steroid will be produced. When P450c17 is absent, such as in the zona glomerolosa, aldosterone is produced. When the 17 α-hydroxylase activity of P450c17 is present cortisol is produced. When both the 17-α-hydroxylase and 17,20 lyase activities of P450c17 are present sex steroids are produced. All steroid hormones are made from pregnenolone produced by P450scc; thus, the presence or absence of each of the activities of P450c17 directs pregnenolone towards its final metabolic pathway.”
-Walter Miller MD – University of California
Now let’s get deeper and think if we upregulate one of the end products do the other end products go down?
The opposite of the “Pregnenolone Steal” would be men going through puberty. At about age 8 to 10 serum DHEA and DHEA-S concentrations rise exponentially but we see no changes in ACTH or cortisol throughout this process.
Hmmm…
Now, we do see is DHEA lowered in chronic disease states, like metabolic syndrome and inflammatory autoimmune disorders, but the mechanism hasn’t been completely hashed out.
“This study indicates that changes of serum levels of DHEA in relation to serum levels of other adrenal hormones are completely different in patients with an acute inflammatory stressful disease state compared with patients with chronic inflammation. The decrease of serum levels of DHEAS and DHEA is typical for chronic inflammation and TNFα and IL-6 play a predictive role for these changes.”
-Straub et al.
BUT, we do know that inflammatory cytokines light up the midbrain and drive sympathetics. Well hi there – Brain.
To me, here is where the theory really absolutely falls apart – testosterone. This is because the bulk of testosterone ISN’T made in the adrenal cortex. In males, man juice is made in our testicles, therefore pregnenolone for its production could not be getting eaten up by cortisol production as this whole process starts over in the testicles themselves.
For females, especially in and around menopause most of their sex steriods are derived from DHEA, thus if the HPA axis is completely dysregulated we know they are not going to do well in this time frame.
Now, I just want to look at the numbers involved in testing.
Serum cholesterol is in units of mg/dL
Testosterone ng/dL
Cortisol ug/dL
DHEA-S ug/dL
1mg/dL = 1,000,000 ng/dL
1mg/dL = 1,000 ug/dL
So if I make a fictional man with normal/optimal hormonal values named the Mountain. He would have around 250-350,000 times more cholesterol in his blood than testosterone, 10,000 times more cholesterol than cortisol, and 500 times more cholesterol than DHEA-S.
Thus, from a substrate availability context, there is just too much cholesterol around for that to be the limiting factor in any of this and from the hardcore science guys and gals we know it isn’t.
Now if you take into account that every one of those teaching the “Pregnenolone Steal” seem to be doing so off results from salivary testing, this gets even more tumultuous as salivary hormones represent bioavailable hormone fractions, not actual production. Additionally, the units get fuzzier and my head hurts.
In saliva – cortisol is in nmol/L and DHEA is in ng/mL
1ug/dL = 27.5 nmol/L
1ng/mL = .1 ug/dL
To throw another wrench in salivary testing we have a relatively new field (late 1980s) called intracrinology where peripheral tissues have been found to make sex steroids from circulating DHEA.
Thus, even though salivary hormones have been found to be correlated with serum, what are these salivary panels really measuring and can we really infer anything about actual adrenal production from these values?
My hunch is No.
Also, as I have laid out in previous posts, these numbers tend to have a very high intrasubject day to day variability.
The point of this article was to show you that this is much more complicated than THIS steals THAT and if we look at the rate limiters these processes seem to be regulated by two things the brain and mitochondrial health.
“Age-related declines in steroidogenesis are caused by a global reduction in steroidogenic enzyme gene expression and by decreases in the rate of cholesterol transfer to mitochondria. In fact, the senescence of Leydig cells is involved at all aspects of the steroidogenic process, from LH binding to the steroidogenic reactions in the smooth endoplasmic reticulum. Reactive oxygen species, produced as a by-product of steroidogenesis itself, may be responsible for age-related reductions testosterone production.”
-Matthew Hardy PhD
In an attempt to lasso a bow around all this, if we want the hormonal orchestra of our bodies to not sound like a brigade of 6 year old tuba players on LSD, we have to master the fundamentals, protect our mitochondria, get rid of underlying sources of inflammatory cytokines, and do everything in our power to live in a more parasympathetic state so that all of these hormonal axes don’t get jack-knifed at the level of the brain because from the research it looks like this is much bigger than a substrate availability issue.
References:
- Straub RH, Lehle K, Herfarth H, Weber M, Falk W, Preuner J, et al. Dehydroepiandrosterone in relation to other adrenal hormones during an acute inflammatory stressful disease state compared with chronic inflammatory disease: role of interleukin-6 and tumour necrosis factor. European journal of endocrinology / European Federation of Endocrine Societies. 2002 Mar;146(3):365-74. PubMed PMID: 11888843.
- Labrie F. Adrenal androgens and intracrinology. Seminars in reproductive medicine. 2004 Nov;22(4):299-309. PubMed PMID: 15635498.
- Labrie F. Intracrinology in action: importance of extragonadal sex steroid biosynthesis and inactivation in peripheral tissues in both women and men. The Journal of steroid biochemistry and molecular biology. 2015 Jan;145:131-2. PubMed PMID: 25240498.
- Luu-The V, Labrie F. The intracrine sex steroid biosynthesis pathways. Progress in brain research. 2010;181:177-92. PubMed PMID: 20478438.
- Qiang Dong MPH. Leydig Cell Function in Man. 2004. In: Male Hypogonadalism [Internet]. Contemporary Endocrinology; [23-43].
- Payne AH, Youngblood GL. Regulation of expression of steroidogenic enzymes in Leydig cells. Biology of reproduction. 1995 Feb;52(2):217-25. PubMed PMID: 7711191.
- Ye L, Su ZJ, Ge RS. Inhibitors of testosterone biosynthetic and metabolic activation enzymes. Molecules. 2011;16(12):9983-10001. PubMed PMID: 22138857.
- Auchus RJ. Overview of dehydroepiandrosterone biosynthesis. Seminars in reproductive medicine. 2004 Nov;22(4):281-8. PubMed PMID: 15635496.
- Miller WL. Androgen biosynthesis from cholesterol to DHEA. Molecular and cellular endocrinology. 2002 Dec 30;198(1-2):7-14. PubMed PMID: 12573809.




Recent Comments