This post is brought to you by Coach Adam Estrin of Pepperdine University.
Part I: Docosahexaenoic Acid.
Do you even fish oil, bro?
At this point, it’s no secret that long chain polyunsaturated fatty acids (LCPUFAs) are ubiquitously involved in cellular processes and are the chemical backbone of all cell membranes, particularly membrane-rich tissue like gray matter. The entire totem pole of research has piled on evidence since the early 80’s that invokes that DHA in particular has a laundry list of potential benefits from cardiovascular health to visual and cognitive enhancement.
The global market for DHA-rich fish oil boasted $1.9 Billion in 2018 and is expected to eclipse $3 Billion in 2025 (1). The ensuing review will attempt to take an objective step back from the hoopla, and weasel our way deeper into the weeds of the mechanisms at play.
Specifically, we will examine the role DHA consumption is thought to play in neurocognitive development in infants.
While DHA is abundantly involved in our biochemistry, objectivity and speculation toward existing literature leads to some pretty…. fishy conclusions.
DHA and its Evolutionary Impact
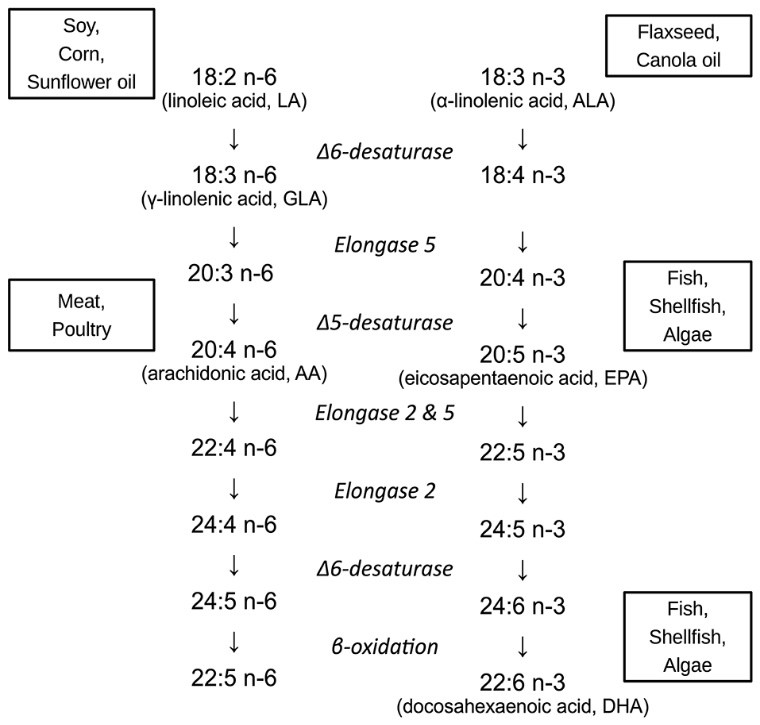
Docosa (22 Carbons) Hexa (6 Double bonds) Enoic Acid, is the most elongated and unsaturated fatty acid found in nature. As an essential fatty acid (cannot be synthesized endogenously), DHA must be consumed in our diet, specifically through seafood.
22:6 = 22 Carbons, 6 Double Bonds. N-3 = Omega-3. ALA has been shown to convert to EPA within pregnant women. EPA will not convert to DHA in sufficient quantities endogenously, which is why we rely on DHA intake through seafood (7).
DHA was a key structural component of cell membranes 600 million years ago and has been since preserved within neural signaling systems (2). Despite the existing paradigm of early Homo sapiens tracking down wildebeests in savanna lands with complex tools, emerging evidence puts early hominids on coastlines in marine environments amid much more accessible and abundant sources of seafood rich in DHA (3). This more intuitively sound story is also boosted by evidence that suggests that this vast LCPUFA influx coincided chronologically with rapid brain growth in early humans (4). While speculative, it is pertinent to consider that our heavy reliance on DHA and other LCPUFAs were mechanistically relevant in the protection and development of the human brain.
Survival of the Fattest
In this new light, the conservation of a costly, vulnerable brain throughout thousands of millennia is demonstrated in (wait for it…) fat babies. Not phat, literally fat. Some may look at the chunky little cheeks as an evolutionary ploy for cuteness and thereby parental care – but in reality, subcutaneous adipose tissue is a key fuel reserve for neurological development in infants when food intake is limited in meeting energy demands.
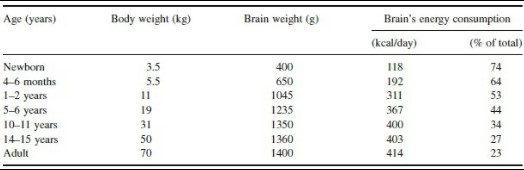
Throughout the first few weeks of gestation, infant development is largely influenced by the mother’s diet. In the third trimester, the placenta runs the show and pumps DHA and other selected nutrients into the fetus. At birth, the fetal brain consumes more than 70% of dietary energy to allow for ensuing neurological development (5).
This makes sense, when considering that cognitive impairments in preterm infants, who miss out on fat deposition and the key transfer of DHA in the third trimester, occur between 20% – 64% of the time (6). These neurological shortcomings also prevail in infants who were fed non-DHA nutrient formulas instead of DHA-rich breast milk post-natally.
(4) Average energy consumption compared to brain and body weight from birth to adulthood. 74% in newborn infants, yikes!
Part II: Research, Yuck!
Well, we’ve made our case. DHA has a 600 million year evolutionary impact and remains as a key fuel reserve in supporting normal neurological development in infants. This concept is vigorously favored among literalllyyy thousands of randomized controlled trials (RCTs) and systematic reviews.
However, once you get past the shiny abstracts and done-up conclusions, therein lies the meat n’ potatoes – what’s within the study methods and designs?
Klevebro
Klevebro et al. published an extensive systematic review in 2020 that objectively dissected major player studies to date. The standardized assessment tool in the majority of neurological outcome studies is the Bayley Scales for Infant Development (BSID). A key testing parameter within the BSID, is the Mental Development Index (MDI). Klevebro et al. determined that in order to obtain 85% power accompanied by a 95% confidence interval to detect a four-point difference in MDI, 253 infants would be required in both the control and intervention groups for the data to show clinical significance (7).
With respect to this newfound parameter, the vast majority of existing studies that rely on the BSID as its outcome determinant are inherently flawed, based on an underpowered sample size. While we shouldn’t throw the baby out with the bathwater (heh), this does add a constraint to the mix we must take into account.
Control Yourself
Also, the randomized controlled trials (RCTs) utilize different doses, test at different durations, and target different outcome measures. Linoleic Acid (LA), is an 18-Carbon Omega-6 Fatty Acid used in the control formulas in a ton of RCTs. It is thought that LA competes mechanistically for the same enzymes that metabolize DHA. Thereby, LA content in the control formulas would inherently confound outcome measures. In the comprehensive studies to date, LA content has varied from 0-20%, which could conditionally skew findings (7).
Klevebro’s review evaluated control formulas with ratios that ranged from 5:1 to 15:1, a range that could diversify outcomes. With so much variance in the control formulas, and dispersed targeted outcome measurements, it is tough to make a succinct, concise argument toward the efficacy on one specific outcome.
Let’s Have an Intervention
DHA contribution in intervention formulas varied anywhere from 0.2-0.3% of total fatty acids, to as high as 1% in a few studies. Makrides et al. compared dosages in a controlled trial of a 0.3% versus 1% DHA intervention formula. Both solutions were administered to infants between day 2 to 4 of birth until 18 months aaaand at 18 months… they found no difference in cognitive outcomes (10).
Additionally, a DINO trial controlled for DHA dosage in the intervention formula with 1% and 0.3% groups. At the conclusion of the trial, there was no difference in overall Bayley Scales of Infant Development scores at 18 months, but the Mental Development Index of the high dose group was slightly elevated in girls. Although, subsequent tests at 3-5 and 7 years showed once again no difference in behavior, attention, or IQ (11).
Maternal Diet
Food production within the past 100 years has changed drastically. We can thank the industrial revolution of the nineteenth century for the ability to infuse seed oil additives into pretty much all of the processed food we consume. Soy oil is responsible for 20% of the calories consumed in America (12).
It is speculated that at one time in our prehistoric diet the ratio of Omega 6:Omega 3 was nearly 1:1. We also believe that this 1:1 ratio is also present in cerebral cortex phospholipids in infants one month after birth (13). However, the product of aging and our modern Western diet has distorted these ratios to as high at 20:1 in adulthood.
It seems as though the maternal diet during pregnancy and lactation, with respect to these ratios, can have huge implications on DHA transfer from mother to infant and provides as another variable that can challenge the polished study titles and abstracts.
Part III. Bringing it Home
In summary, it seems as though the mechanisms are hairier than they first appeared. Extraneous factors, intricacies, and variance have limited a lot of claims made by these studies. How essential DHA consumption is in neonatal neurological development remains to be seen. However, this is expected. Research across disciplines will always have limitations, but it is still our best tool to guide practice. Not going to reuse the same pun but something about babies and bathwater.
Given the evident and vast role DHA plays biochemically, and our biological shortcoming to synthesize DHA endogenously, oyster happy hours seem like a pretty good idea. While more research needs to be performed, there seems to be asymmetric risk (as Eric Helms puts it) in consistent DHA consumption. Low risk, high potential upside.
Research has for the most part dispelled the panic behind mercury and inorganic compounds in seafood. Limit large predators (swordfish, king mackerel, tilefish, shark), but take the green light on little oily guys (sardines, anchovies, salmon). The benefits of 2-3 servings per week far outweigh the costs (14).
In regard to neonatal development, DHA consumption appears profoundly more imperative in preterm infants as they miss out on the placental exchange of nutrients in the third trimester.
With DHA being processed out of our food supply and seed oils being pumped in, this could potentially affect chemical compounds in brain tissue and resulting neurological development in infants. It is estimated that 30% of LCPUFAs in breast milk are highly influenced by diet, while 70% come from adipose storage and endogenous synthesis (15). Low maternal stores combined with a high influx of processed food could potentially result in adverse effects in infants (16).
So, next time you’re at the grocery store, get yoself some deliciously scrumptious, wild-caught, canned sardines. Just be mindful of where/when you crush them – people won’t care that you’re being health-conscious but they will care about the smell.
REFERENCES:
- https://www.marketwatch.com/press–release/fish–oil–market–2019–global–industry–size–demand–growth–analysis–share–revenue–and–forecast–2025–2019–05–27
- Stephen C. Cunnane. Human Brain Evolution (p. 17). Wiley. Kindle Edition.
- Cunnane, S.C. 2005. Survival of the Fattest: The Key to Human Brain Evolution , River Edge, NJ: World Scientific Publishing .
- Stephen C. Cunnane. Human Brain Evolution (p. 34). Wiley. Kindle Edition.
- Crawford, M.A., Cunnane, S.C., AND Harbige, L.S. 1992. A new theory of evolution: Quantum Theory. In Essential Fatty Acids and Eicosanoids. Invited Papers from the Third International Congress , ed. A. Sinclair and R. Gibson, pp. 87-95. Champaign, IL:AOCS Press.
- Serenius F, Ewald U, Farooqi A, Fellman V, Hafström M, Hellgren K, et al. Neurodevelopmental outcomes among extremely preterm infants 6.5 years after active perinatal care in Sweden. JAMA Pediatr.(2016) 170:954–63. doi: 10.1001/jamapediatrics.2016.1210
- Front. Pediatr., 10 January 2020 | https://doi.org/10.3389/fped.2019.00533
- Lancet.2007 Feb 17;369(9561):578-85
- Lozada LE, Desai A, Kevala K, Lee J-W, Kim H-Y. Perinatal brain docosahexaenoic acid concentration has a lasting impact on cognition in mice. J Nutr.(2017) 147:1624–30. doi: 10.3945/jn.117.254607
- Makrides M, Neumann MA, Jeffrey B, Lien EL, Gibson RA. A randomized trial of different ratios of linoleic to alpha-linolenic acid in the diet of term infants: effects on visual function and growth. Am J Clin Nutr.(2000) 71:120–9. doi: 10.1093/ajcn/71.1.120
- Smithers LG, Collins CT, Simmonds LA, Gibson RA, McPhee A, Makrides M. Feeding preterm infants milk with a higher dose of docosahexaenoic acid than that used in current practice does not influence language or behavior in early childhood: a follow-up study of a randomized controlled trial. Am J Clin Nutr.(2010) 91:628–34. doi: 10.3945/ajcn.2009.28603
- Stephen C. Cunnane. Human Brain Evolution (p. 71). Wiley. Kindle Edition.
- Svennerholm L. Distribution and fatty acid composition of phosphoglycerides in normal human brain. J. Lipid Res. 1968;9:570–579
- Public Health Nutr. 2018 Aug; 21(11): 2149–2159. Published online 2018 Mar 26. doi: 10.1017/S1368980018000599
- Koletzko B., Rodriguez-Palmero M., Demmelmair H., Fidler N., Jensen R., Sauerwald T. Physiological aspects of human milk lipids. Early Hum. Dev. 2001;65:S3–S18
- Koletzko B. Trans fatty acids may impair biosynthesis of long-chain polyunsaturates and growth in man. Acta Paediatr. Scand. 1992;81:302–306.


Recent Comments